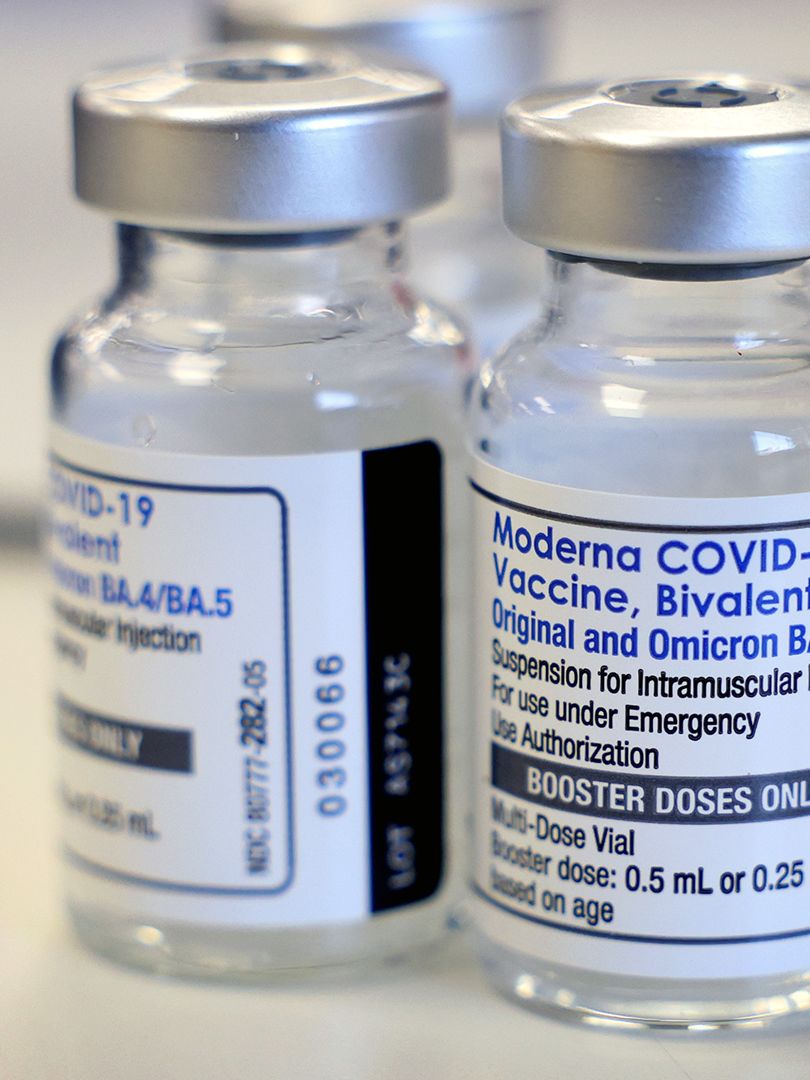
The U.S. Food and Drug Administration amended the terms of its emergency use authorizations for the Pfizer and Moderna bivalent vaccines on Tuesday, allowing people ages 65 and older and certain people with weakened immunity to get additional doses before this fall’s vaccination campaigns.